At Progress Clinical Research, we believe that efficient, high-quality clinical trials accelerate the development of innovative treatments, ultimately enhancing the health of many. We focus on Phase 1b, 2 and 3 trials to help bringing safe, effective treatments to patients.
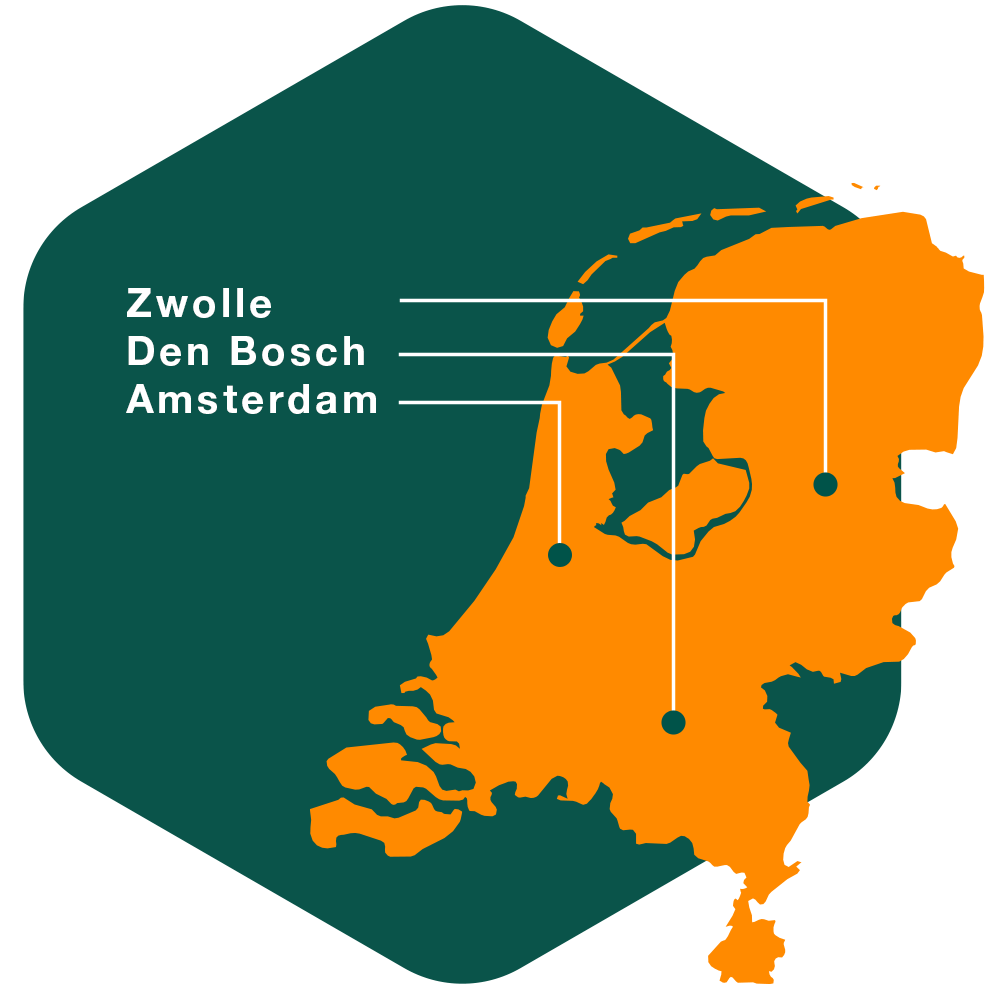
With three integrated trial sites in the Netherlands, our centralized model combines specialized teams and experienced oversight, ensuring standardized processes, seamless communication, and high-quality trial execution for reliable healthcare results.